Imaging Services for Clinical Trials
Innovative Global Imaging Services for Oncology Clinical Trials
Accelerate the development of life-saving cancer treatments with Median’s best-in-class clinical trial imaging services for oncology clinical trials.
How Median’s Clinical Trial Imaging Solutions Support Research and Development
As a global market leader in oncology clinical trial imaging, Median iCRO helps life science companies transform imaging data into life-changing insights. Our innovative tools and techniques, including AI and machine learning revolutionized the use of medical images in clinical trials by extracting the highest quality imaging biomarkers – setting a new standard for developing new therapies.
Leverage strategic AI-driven insights and make better decisions, earlier
We help sponsors worldwide gain a competitive advantage in the high-stakes world of clinical development – from getting trials up and running, to delivering life-changing treatments to market faster.
Identify the most promising compounds early with Median’s intelligent clinical trial imaging technologies and scientific expertise.
Watch our video to discover more about who we are and the innovation that drives how we can help you deliver groundbreaking medicines to patients that need them the most.
Why Median for Clinical Trial Imaging?
Median iCRO is the gold standard for oncology clinical research imaging in the life science industry, helping you maximize value and reduce risks.
Rely on a leader
- Median iCRO is a trusted leader in clinical trial imaging, with more than 20 years’ experience and a wealth of oncology and clinical expertise.
- We pioneered Artificial Intelligence (AI) in clinical trial imaging and have supported hundreds of trials globally.
- Our innovative, intelligent technology delivers vital imaging insights, analyses, and data to help shape clinical development for some of the world’s top pharma companies.
Ensure operational excellence
- Dedicated experienced project management teams bring transparency and proactive support to every project.
- From study start up to study close, you can count on our teams to be with you throughout your trial. Count on us for Central Reviews, Collect and Holds, Retrospective Analyses and more.
- Median iCRO’s configurable software and flexible workflows are customized to align with your operations and match your study needs. Our commitment to quality drives everything we do.
Boost R&D efficiency
- Use AI and machine learning to extract new insights to inform your drug development strategy.
- From electronic image transfer to metrics-driven analysis and scientific domain expertise, Median offers best-in-class solutions and resources for oncology clinical trial imaging.
- Maintain accuracy and consistency in image reading and glean key development insights at every stage of the product lifecycle.
Build Confidence and Trust with Median’s Proven Clinical Trial Imaging Innovation
Harness the power of Median’s iCRO clinical trial imaging solutions to amplify your image management and drive efficiency from study start up to submission.

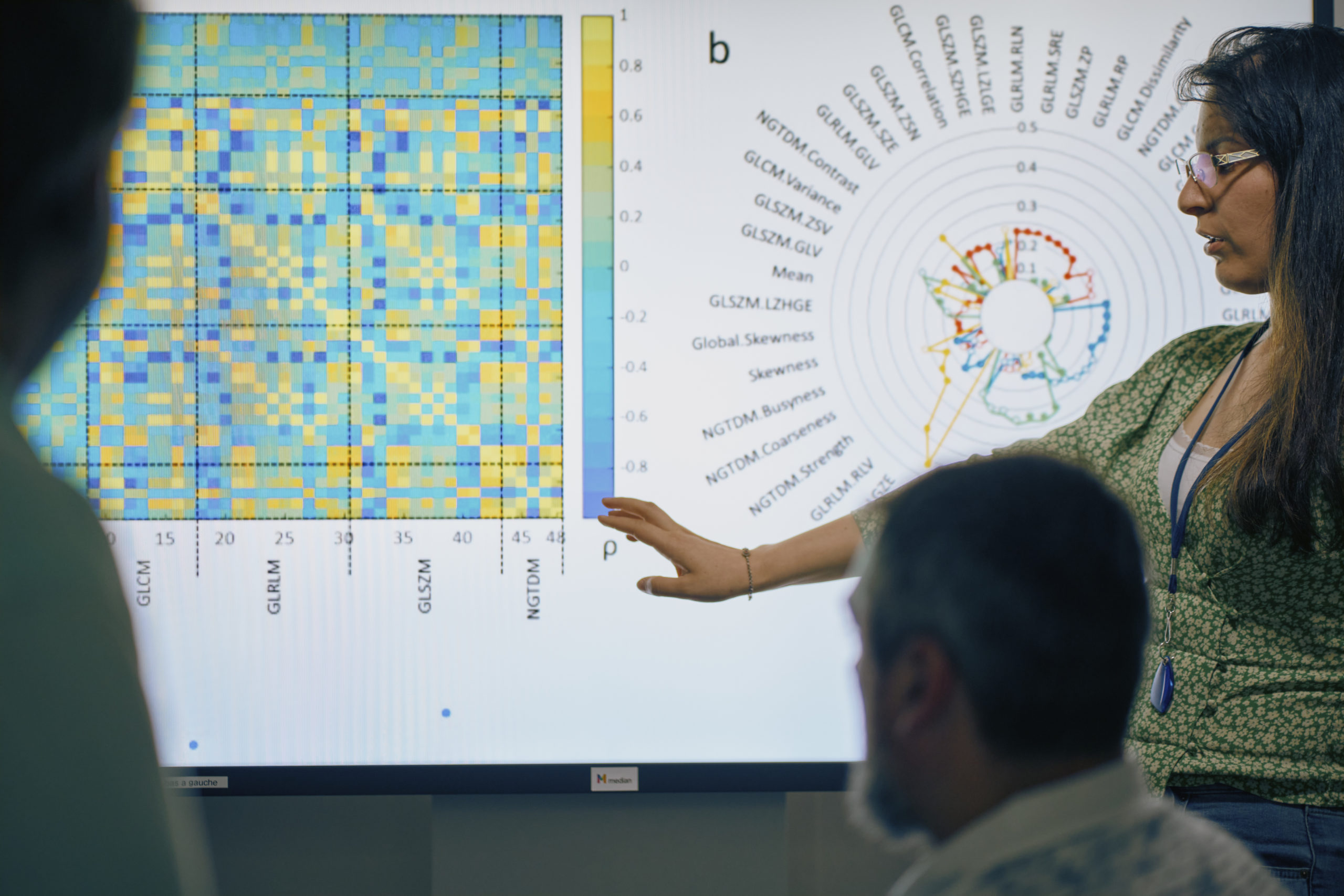
Imaging Lab
Leverage more insightful medical imaging data with our new transformative solution.
Using AI, ML, data mining and radiomics we help you extract much more from clinical trial images than the industry has ever seen before.
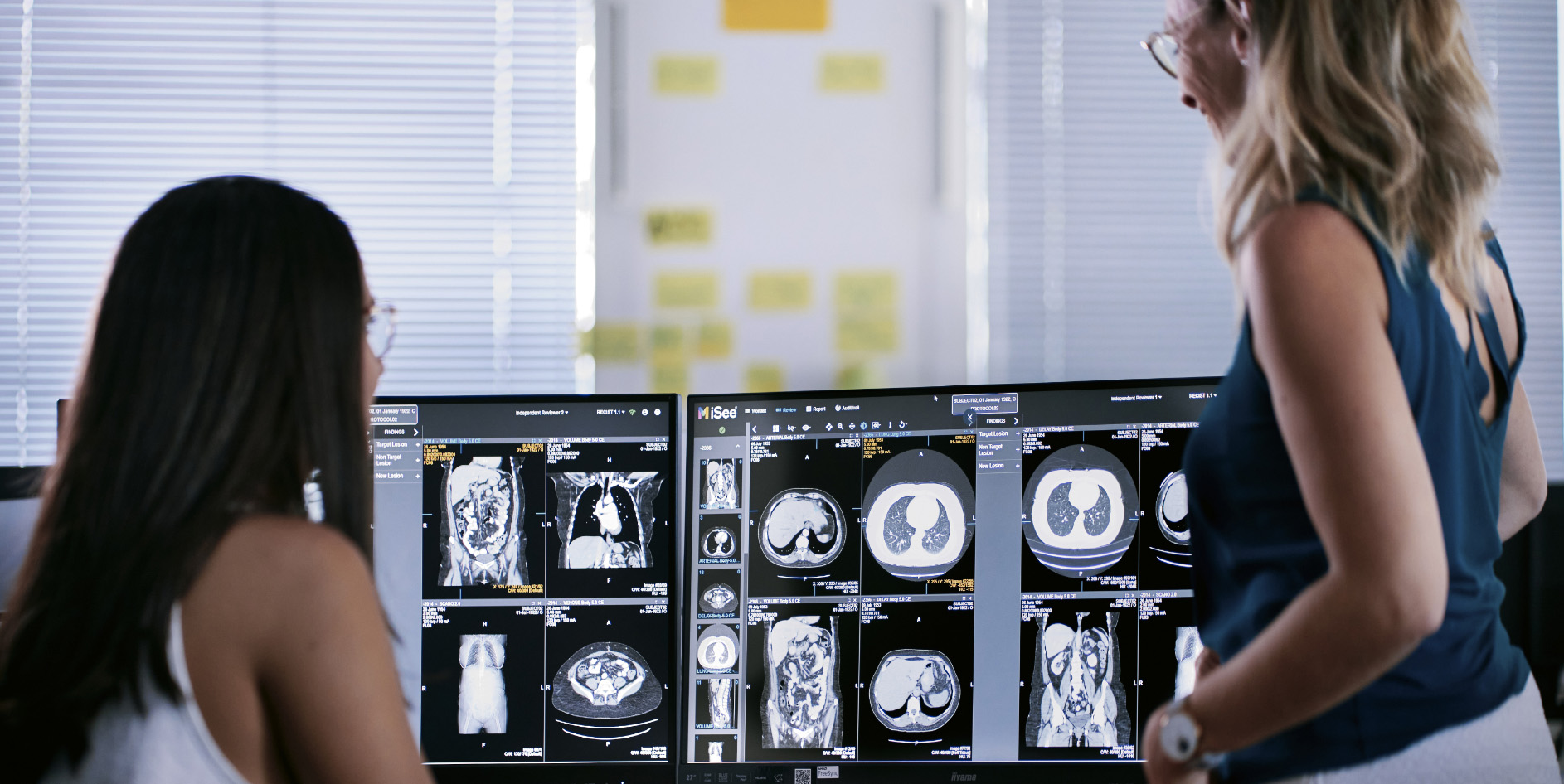
iSee® Viewer
Gain expert oversight of clinical trial images with our proprietary, flexible software that helps identify, quantify, and track lesions across all time points.
Reduce human error and cover a wide range of solid tumors in cancer indications.

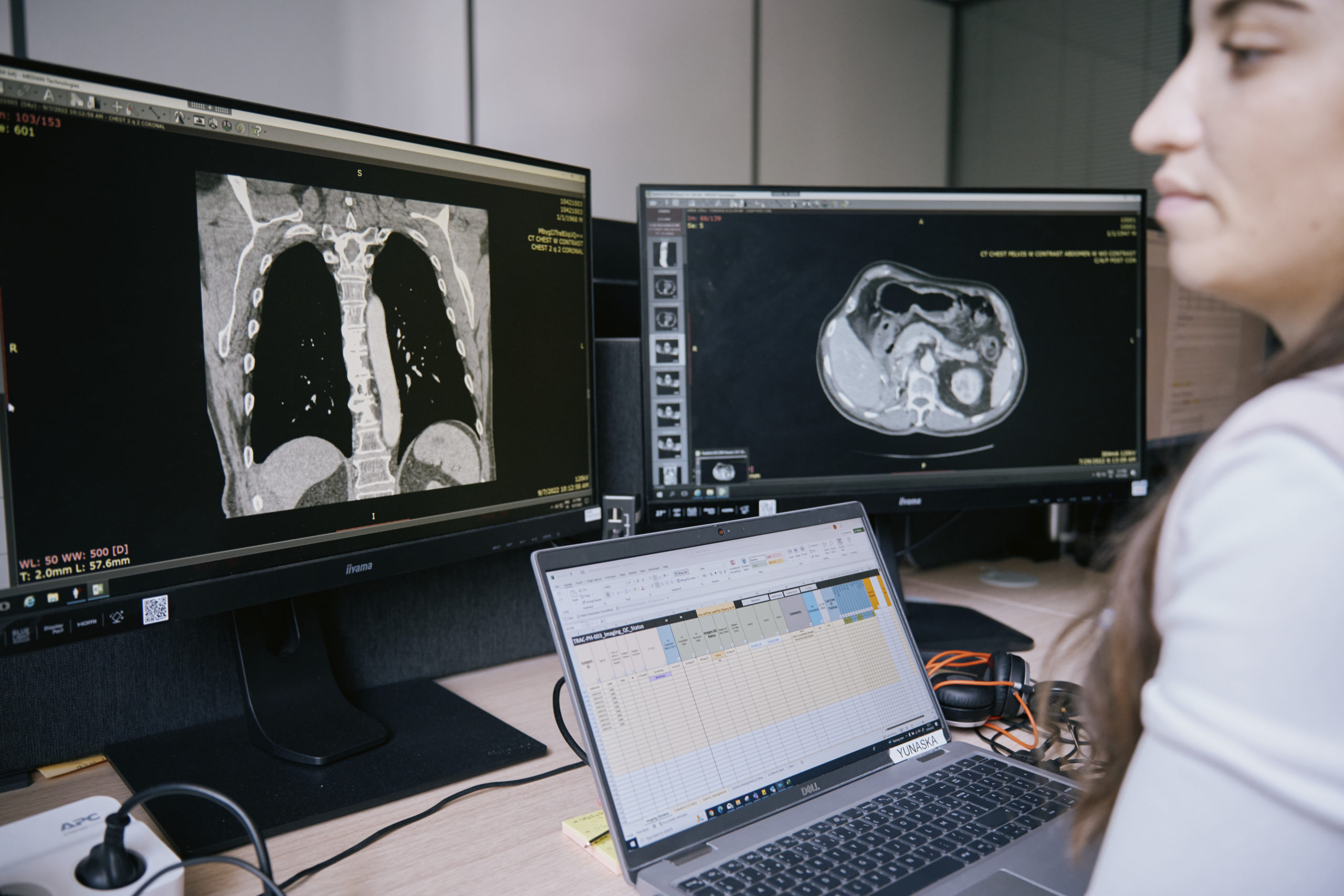
iSee® Portal
Real-time, robust clinical trial image reporting at your fingertips.
From site management, image collection, data management and reads, our reporting boosts operational performance, manages risks, and keeps you on top of your study’s imaging workflow.
Explore our services across the development continuum
Click to learn more
Scientific Expertise
Looking for an oncology team of experts who share your passion and can deliver valuable insights on the clinical landscape for your compound? Median iCRO’s lead scientific experts, medical, and study teams can provide know-how on protocol design and imaging criteria selection. Our experienced teams are dedicated to helping you be successful and are true experts in oncology clinical trial imaging.
AI-driven Clinical Trial Imaging for the Life Sciences
Intelligent imaging is fast becoming a necessity for driving successful clinical trial insight.

Putting advanced analytics to work
Median iCRO’s purpose-built technologies and scientific domain expertise put advanced analytics to work, including artificial intelligence (AI), machine learning (ML) and automation, helping you:
- Get more value from your research and development strategy
- Identify patients with early-stage cancers
- Derive key imaging insights and identify critical actions
- Leverage significant learnings to improve decision making
- Accelerate time to insights and market
Median iCRO offers unprecedented clinical trial imaging expertise and partners with Pharma, Biotech and CROs globally, helping to shorten timelines, decrease costs and maximize asset values.
Pharmaceutical
Innovation and helping solve cancer is what we are focused on at Median. Our commitment to innovation in the oncology clinical trial imaging space is what keeps us up at night. Depend on us to help you with unprecedented analytical clinical trial imaging insights – to improve business and patient outcomes, earlier, rather than later.

Leading organizations are embracing the value of non-invasive medical Imaging to correlate radiological signatures with underlying molecular pathways and patient outcomes. Median is leading the charge with AI-powered imaging data and analyses enabling our customers to focus on identifying promising compounds earlier, and with more precision.
Yan Liu, MD, MSc, PhDChief Medical Officer,
Median Technologies
Discover our Latest Thinking
BROCHURE
Introducing Median Imaging Lab
Get more value from your oncology trials with transformative
image insights, analyses and more
Brochure
Imaging in Oncology Trials
Delivering best-in-class imaging services and expertise to clinical sponsors worldwide
Scientific publication
Challenges To Assess Bone Metastases In Blinded Independent Central Review (BICR) Of Breast Cancer Trials Using Recist 1.1
ARTICLE
Business Intelligence-based reporting optimizes the operational efficiency of clinical trial imaging services
Explore what we can do for you to help improve the lives of cancer patients and their families.
Median iCRO
intelligent. insightful. innovative.
Where valuable clinical trial imaging data and analytics intersect with advancing human health.
